The Value Proposition
The past 18 months of SARS-CoV-2 has confirmed yet again that Emerging Infectious Diseases (EIDs) threaten human life, health, and economic prosperity. Furthermore, the SARS-CoV-2 Delta variant with its resulting new cases and deaths rivaling our previous peaks has further reaffirmed the threat. EIDs are among the highest security risks facing the world today. And as global populations move and expand, so too will infectious zoonotic disease outbreaks. Global outbreaks are occurring in new locations as shown by the first Ebola outbreak in more than 25 years in Cote d’Ivoire and West Africa’s first-ever case of Marburg virus disease confirmed in Guinea. Our defense is improving, but we need an offense too. We need to stop epidemics from becoming pandemics.
Our offense must include a full arsenal of medical countermeasures, stockpiled and ready to deploy anywhere in the world when an outbreak occurs. A portfolio of human monoclonal antibodies is a necessary weapon in that arsenal – in addition to diagnostics, vaccines, and other therapeutics – to provide immediate prophylactic immunity to affected communities.
The Global Pandemic Prevention and Biodefense Center (GPPBC or “the Center”) will help prevent future outbreaks from becoming pandemics by developing a stockpile of human monoclonal antibodies in advance for emerging infectious diseases, and by integrating antibody distribution and delivery across the global health and pandemic prevention ecosystem.
“Outbreaks are inevitable, but pandemics are optional.”
— Dr. Larry Brilliant, renowned epidemiologist, technologist, philanthropist, and author
Executive Summary

The need for new pandemic prevention and response solutions is more urgent now than ever. SARS-CoV-2 has now claimed more than 4 million lives and is projected to cost global economies more than $28T. Those grim statistics will continue to rise as the world suffers from the SARS-CoV-2 Delta variant and its resulting new cases and deaths rivaling our previous peaks. In addition, global outbreaks of other Emerging Infectious Diseases (EIDs) are occurring in new locations as shown by the first Ebola outbreak in more than 25 years in Cote d’Ivoire and West Africa’s first-ever case of Marburg virus disease confirmed in Guinea. The global response to SARS-CoV-2 has been reactive, lacking in coordination, limited in solutions for at-risk populations, and has not taken full advantage of the spectrum of solutions available. The time is right to create and fund a new public-private partnership that fills a critical gap in global preparedness to contain epidemics before they become pandemics.
Key founders and supporters of the Center include leaders from the scientific community, philanthropy, state and local government, academia, industry, and global public health. The Center has received initial funding from the S&R Foundation, the Bill & Melinda Gates Foundation (BMGF), the Coalition for Epidemic Preparedness Innovations (CEPI), the State of Maryland, Montgomery County, and various industry participants. In addition, the Center has convened a cross-sector Steering Committee comprised of over 40 highly respected senior executives, scientists, and policymakers to provide scientific and strategic direction.
The Center will officially launch in the second half of 2021 to accelerate the delivery of monoclonal antibody (mAb) solutions with its flagship initiative, Advancing Human Epidemic Antibody Defenses (“AHEAD100”). The compelling vision and scientific leadership for the AHEAD100 initiative are provided by Dr. James Crowe, a globally renowned mAbs and infectious disease specialist and Chief Scientist for the Center. Through this program, the Center will coordinate across government, private sector, and civil society to provide a stockpile of monoclonal antibody medical countermeasures and integrate these solutions across the pandemic prevention ecosystem. Over the next six years, this ambitious program will develop and stockpile mAbs for the 100 pathogens across 15-25 pathogen families most likely to result in pandemic potential outbreaks.
The primary benefits of mAb medicines include speed, safety, efficacy, cost, trust, and time. Antibodies can now be developed for the most likely Emerging Infectious Diseases (EIDs) and stockpiled in advance for outbreak response, as the time required to identify, test, manufacture, and deliver mAbs is rapidly decreasing. The development of mAbs also enables the development of diagnostics and vaccines based on the same protein antigens. Upon injection, mAbs provide “instant immunity” to protect frontline healthcare workers from infection. These same mAbs can be used therapeutically as well as to prevent infection after exposure.
AHEAD100 will take a portfolio approach that capitalizes on advances in mAb development technologies to create safe, effective medical countermeasures that can be rapidly deployed to contain outbreaks. Pathogen targets are aligned with those of global organizations, including WHO, NIH, CDC, and CEPI. This alignment evaluates pathogen characteristics such as transmissibility, virulence, and status of existing therapies under development.
AHEAD100 will help facilitate greater integration and collaboration for biodefense. As both public health and defense communities share interests in pathogen targets and the corresponding solutions, the governance and operating model of the Center will incorporate the respective DoD and HHS communities. The Center will also coordinate with the Bipartisan Commission on Biodefense and the Center for Emerging Infectious Diseases to align specific recommendations and actions. Biodefense requirements are rapidly rising and will be addressed in an integrated manner.
AHEAD100 is structured around the three priorities of PREPAREDNESS, INNOVATION, and RESPONSE. These pillars drive the key activities for building the key elements of the AHEAD100 program, including the stockpile, development, full-lifecycle innovation (including production), distribution, and global response coordination and delivery.
The Center will accelerate pandemic PREPAREDNESS by developing a stockpile of “warm-ready” mAbs through its AHEAD100 program. The target product profile will be highly potent mAbs that have a long half-life, are intramuscular (IM) administered (or other non-infusion delivery methods), and can protect people from infection for a minimum of 6 months. The stockpile of mAbs will address known and emerging pathogens among several bacterial and viral families. These drugs are to be developed and tested through Phase 1 clinical trials, after which the Center will establish global stockpiles.
The Center will drive INNOVATION throughout the infectious disease ecosystem by facilitating better collaboration across public health and defense, the selection of specific R&D platforms, advancing routes of dosage administration (intramuscular vs. infusion), enhancing manufacturing capability and capacity, lowering costs, and increasing efficiency. The Center will also accelerate a rapid response mAbs development capability to better respond to newly identified or emerging pathogens (Disease X) by delivering new mAbs countermeasures at speed.
Finally, the Center is already working with the appropriate government agencies and is establishing partnerships with key entities in the pandemic response chain, including CEPI, FIND, Rockefeller Foundation, and WHO, to enable a coordinated rapid RESPONSE to public health emergencies, and which govern how mAbs will be used in during outbreaks. This includes marshaling the regulatory ecosystem to support the use of a stockpile in emergencies with speed and effectiveness. In addition, the formation of critical partnerships amongst upstream providers in surveillance and diagnostics and downstream providers in vaccines and antiviral solutions will also be integral.
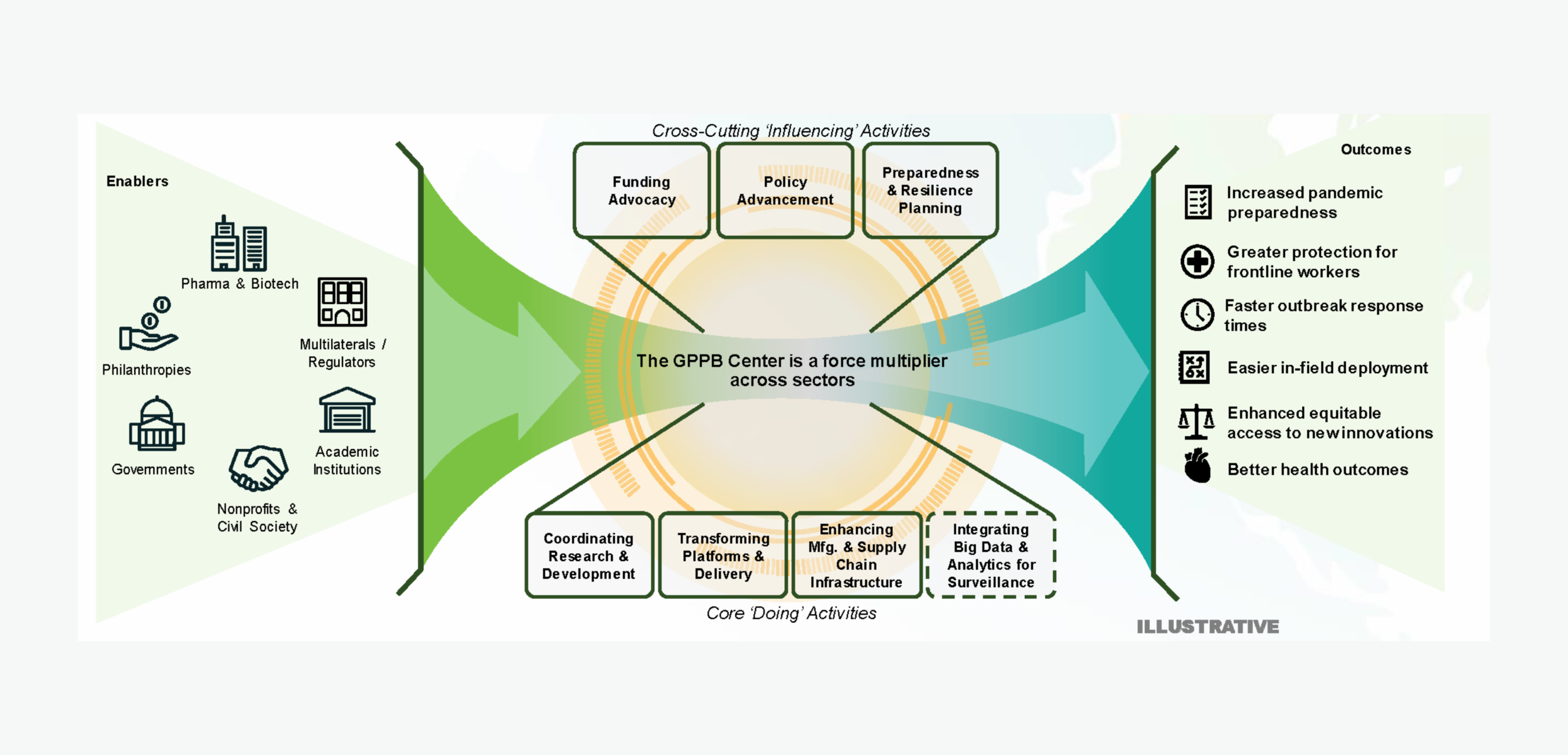
Figure 1: The Center will fill a critical pandemic prevention and response gap, collaborating across a public-private ecosystem.
With global coordination across the pandemic response ecosystem, the Center will address the need for rapid response prophylactic and therapeutic solutions for children, elderly, immunocompromised, minority, and other vulnerable populations. This emphasis on the most vulnerable people will be enabled by the integration of new medical countermeasure development with last-mile delivery and training efforts to provide better community and healthcare system response.
The Center seeks to raise $2.5B from government and global philanthropy to implement a 6-year strategic plan. Initial funding will support capacity building complemented by R&D programs to develop the initial antibody solutions through clinical trials, manufacturing, capability demonstrations, and stockpiling.
Background

Outbreaks and pandemics have been a constant companion throughout human history, with growing populations and dense urban centers furthering the associated risks. In the past twenty years alone, the world weathered SARS and MERS as ominous foreshadowing for SARS-CoV-2. Regrettably, over that time, the global community has not learned to adapt to the increasing recurrence of disease caused by pandemic-potential pathogens, even with the modernization of medicine and the availability of new process innovations.
Coordination breakdowns often become apparent across ecosystem participants where siloed capabilities generate insight without integrated actions. For example, in the case of SARS-CoV-2, scientists knew that coronaviruses were circulating in bats well before 2019. Despite this, there were limited changes to wildlife regulation policies, and surveillance networks were not bolstered. Once SARS-CoV-2 emerged, the world initially relied on rudimentary measures like social distancing and “masking up” to avoid further transmission.
However, there is good news in looking to the future: the advent of monoclonal antibodies (mAbs) has enabled the global public health community to improve rapid response and containment. By incorporating mAbs into a comprehensive rapid response strategy, the global public health community can increase healthcare system resilience across the globe and arrest disproportionate damage to frontline workers and vulnerable populations.
The scale of the SARS-CoV-2 disaster has also provided a “call to action” for other positive public health outcomes moving forward: the global community is now rallying around the mission to prevent another pandemic. The “100 Days Mission to respond to future pandemic threats” report (June 12, 2021, sponsored by Sir Patrick Vallance and Melinda Gates, and presented to the G7 pandemic preparedness partnership) lays out a roadmap for achieving a 100 Days Mission to develop safe, effective diagnostics, therapeutics (including mAbs), and vaccines at scale and ready to be deployed equitably within 100 Days of a public health emergency.
The report calls for “…a mission-focused approach to prepare for known risks, through concerted effort and collaboration between the public and private sectors and academia. The pathogens of greatest pandemic potential are represented by respiratory viruses, but we know that viruses from at least 25 viral families can cause human disease, and in theory, the next pandemic could emerge from any of these families. We can, and should, prepare prototype DTVs (Diagnostics, Therapeutics, Vaccines) to treat pathogens of greatest pandemic potential and progress them to a stage that can be adapted quickly to respond to a specific pathogen threat….” The Center’s mission is fully aligned with this call to action.
Further, the 100 Days Mission report calls for expanding CEPI, which currently focuses on vaccines, to provide the R&D funding and coordination of a broader DTV solution set. CEPI is a collaborator in the formation of the Center and supports leveraging the Center’s launch as a mechanism for delivering mAb solutions across CEPI’s global DTV ecosystem.
The initiative to create the Center was generated from the SARS-CoV-2 Strategic Renewal Task Force, a 12-month Connected DMV effort to capitalize on the assets and capabilities in Greater Washington, D.C. to create regional, national, and global programs to provide for a stronger and more resilient society in the aftermath of SARS-CoV-2. In August 2020, Dr. Crowe presented his AHEAD100 vision to the Task Force, who unanimously approved the associated Strategy Phase captured in this document. While the Center is physically located in Greater Washington, D.C., this is a global initiative that fills a critical void in the broader ecosystem for pandemic prevention and preparedness.
In addition, the Center’s strategic location headquartered in the greater Washington, D.C. area will provide proximity to crucial U.S. agencies (NIH, FDA, ASPR/BARDA, etc.) and accelerate pathways to direct federal government funding. Building on BARDA’s successful CARB-X (Combating Antibiotic Resistant Bacteria) program, the Center calls for a similar collaboration model across the public-private ecosystem to fill in gaps.
CARB-X is an innovative public-private partnership funded by BARDA, ASPR, NIAID, the Wellcome Trust, BMGF, and others. The CARB-X Joint Oversight Committee provides governance and investment decision-making and is guided by the funding organizations and key management personnel, including BARDA and NIH/NIAID representatives. A similar U.S. government collaboration model will enable the Center as a nonprofit entity to be funded by government and philanthropic sources with joint oversight and a cross-sector Scientific Advisory Board to advise and guide the AHEAD100 program.
The strategic planning phase activities concluded in August 2021, which resulted in the development of the Center’s Strategic Plan and detailed implementation and financial plans for delivering AHEAD100. During this phase, a Center website (pandemicprevention.org) was launched in conjunction with initial marketing and communications activities. The strategic planning phase was led and overseen by an active and growing Steering Committee of 40 highly respected senior executives and scientists from across the private sector, government, academia, and nonprofit to provide strategic and scientific direction, as shown below.
Figure 2: Steering Committee shaping the Center’s strategic and technical direction (*ex-officio, non-voting).
Our Scope
The Center aims to fill a critical rapid response gap within the pandemic prevention lifecycle. The Center will develop an effective countermeasure that addresses the lack of immediate preventative and therapeutic options between outbreak and containment for all populations. It will coordinate across the surveillance-diagnostics-therapeutics-vaccines ecosystem to deliver an integrated response that uses data, the latest in medical sciences innovation, and partnership/distribution models to effect better health outcomes.
The global community is focused heavily on surveillance, antivirals, and vaccines, which are all needed. Yet mAbs can fill the critical time gap to contain an outbreak in the early days where vaccines cannot, and the Center’s initial focus is on addressing this gap by enabling the creation of a mAbs stockpile. Whereas antivirals provide needed therapeutic treatment to infected persons, the prophylactic benefit of mAbs coupled with their longer duration effectiveness enable mAbs to serve as a bridge to vaccines by providing a means to protect and treat vulnerable populations during outbreaks. Antibodies serve as a safe, effective medical countermeasure to prevent transmission to frontline healthcare workers and directly exposed contacts and caregivers while also treating infected individuals until vaccines are available to immunize broader swathes of the population.
The benefits of mAbs can be realized across all phases within the disease continuum:
Prevention (before exposure)
Post-exposure prophylaxis (after exposure or infection but before the onset of disease)
Therapy of mild disease to prevent hospitalization
Therapy of moderate disease to prevent admission to the ICU
Therapy of severe disease to prevent death
mAbs provide “instant immunity” from infection and can be readily deployed to protect frontline healthcare workers and other post-exposure individuals from infection. Given their inherent safety and efficacy, rapid deployment of mAb medicines during an outbreak will reinforce civilian trust in governments and can preclude civil unrest, travel bans, and trade restrictions, among other harmful societal outcomes. This will help buy the precious time needed to establish diagnostic testing in the field, conduct contact tracing for containment, produce vaccines, and carry out mass vaccination campaigns.
Figure 3: mAbs, antivirals, and vaccines form a complementary ecosystem and can combine to provide a comprehensive pandemic prevention and response toolkit.
Future pandemic potential pathogens are likely to arise from one of approximately 25 pathogen families (details are provided in the appendix). The Center’s AHEAD100 initiative and focus on building a stockpile for at least one or more pathogen(s) in each family affords the pandemic ecosystem an effective medical countermeasure and significantly reduces health system vulnerability and risk. In many cases, the development of one solution accelerates synergistic development across pathogens within a family, as in a recently approved Ebola medicine, Inmazeb.
The target list for the AHEAD100 program aligns with the priorities of the WHO, NIH, BARDA, CDC, CEPI and adds on additional targets, focusing on representative pathogen characteristics. These include transmissibility, virulence, pathogen variants, and the effectiveness of other countermeasures. The scale and extent of the complete list promises to deliver the most comprehensive approach to medical countermeasure preparation to date. The value of a portfolio approach against an emerging or unknown pathogen with little predictability is significant, especially given what we have learned about the devastating effects of pandemics.
The AHEAD100 pathogen list also aligns with public health and national biodefense interests and priorities. The intersection of biodefense threats and public threats calls for collaboration with defense and health agencies in the Center’s prioritization and delivery of mAbs medicines. The Center’s governance will include government agencies in the defense and public health sectors to better facilitate this coordination. AHEAD100 is evaluating the merits of addressing the portfolio of shared targets vs. perpetuating a more opportunistic approach on a case-by-case basis. The Center will also coordinate with the Bipartisan Commission on Biodefense and the Center for Emerging Infectious Diseases to align specific recommendations and actions.
Progressing the development of a broad base of mAbs advances R&D efforts and drives process innovations across the value chain involving a diverse array of participants, from academics, industry players, CDMOs, CROs, and last-mile delivery partners. This results in the creation of a critical “warm ready” capability and delivery engine that can adapt to new pathogens and scale research, development, and industrial capacity to address emerging threats. This ecosystem works well in healthcare delivery for other indications in oncology and immunology. The Center will adopt operating policies aimed at ensuring equitable access to medical countermeasures with the goal to make countermeasures first available to populations when and where they are needed to end an outbreak, regardless of ability to pay.
The Center will serve an integral role in driving the adoption of mAbs within rapid response by driving ecosystem coordination and integration across surveillance, diagnostics, and deployment of medical countermeasures for containment, prevention, and treatment of infected individuals and populations.
The Center will:
Serve as a global facilitator and repository across the pandemic prevention and response ecosystem.
Support and coordinate activities to improve our collective response to epidemic outbreaks across surveillance, diagnostics, mAbs, vaccines, and antiviral solutions, better enable both a national and global response capability, strengthen response capacity in countries at risk, and advance the public health policy and regulatory pathways that govern pandemic-potential diseases.
Facilitate solutions for at-risk populations by working with the scientific, healthcare, and local jurisdictional leaders.
Impact & Strategic Priorities

Our vision is a world without pandemics.
The Center will drive the development of rapid response medical countermeasures and develop partnerships to accelerate our strategic priorities of preparedness, innovation, and response. To achieve these, we will develop a stockpile of mAbs solutions for the top 100 pathogens. We will facilitate innovation by advancing new technologies that improve mAb development speed, efficacy, and costs. Finally, we will demonstrate the effectiveness of the response ecosystem with a capability demonstration that establishes the template for response in an outbreak, gathering data to prove the distribution model and show clinical results.
To prevent future outbreaks from becoming pandemics by providing a stockpile of monoclonal antibody medical countermeasures in advance and integrating those solutions across the global prevention ecosystem.
The Center acts as a facilitator and accelerator that brings together diverse expertise from industry, academia, government, philanthropy, and public health to drive the development of a mAb stockpile. The Center invests in innovation in peripheral areas as necessary to develop efficient, advanced, and reliable solutions. In addition, the Center liaises with national and international agencies to facilitate swift regulatory approvals, enhance global equitable access, and secure the ability to deploy and apply mAbs in any location across the globe.
For AHEAD100, the Center will establish key operational partnerships with academic institutions and industry to drive discovery and pre-clinical results. It will define the selection processes and criteria for organizations with mAbs production capacity (including pharmaceutical companies and CDMOs) and execute agreements to develop mAbs in compliance with regulatory standards necessary to satisfy key regulators, including the WHO, FDA, and EMA.
The Center will create pathways to secure funding, drive policy advancement, and transform regulatory pathways to enhance mAbs adoption in a global public health context. It will take responsibility for the creation and customization of pandemic response protocols pertaining to various entities.
Figure 4: The Center enables research coordination and platform innovation to develop a stockpile of mAbs that facilitates better health and security outcomes.
The impact of these efforts will:
Better prepare governments and public sector stakeholders to become more proactive during outbreaks through the creation of customized and documented scenario plans
Protect our frontline workers, who often suffer disproportionately in medical emergencies – the prophylactic and therapeutic benefits of AHEAD100 mAbs deployment instantaneously shield this vulnerable subgroup, preventing a ripple effect that further weakens healthcare infrastructure during emergencies.
Provide our most vulnerable populations with safe, effective, and tailored solutions that recognize their unique needs
Reduce time to react with a warm-ready stockpile and a manufacturing partnerships network that can be activated as necessary to provide coverage for larger populations as conditions escalate.
Simplify in-field deployment by creating other forms of dosage administration (intramuscular) with lower infrastructure requirements.
Prevent new infections and treat existing infections, mitigating disease severity, and reducing the burden on healthcare infrastructure.
The outcomes above will be enabled through three focus areas:
Figure 5: The Center’s strategic priorities allow the development of a rapid response capability and define plans for its execution in the event of an outbreak.
Priority 1: Preparedness
AHEAD100 will serve as the launch initiative to advance access to safe and effective mAbs against emerging infectious diseases. The advancement of mAbs requires a coordinated effort with active participation by academia, industry, and public health experts. A network of partners is currently assembled and coordinating accordingly.
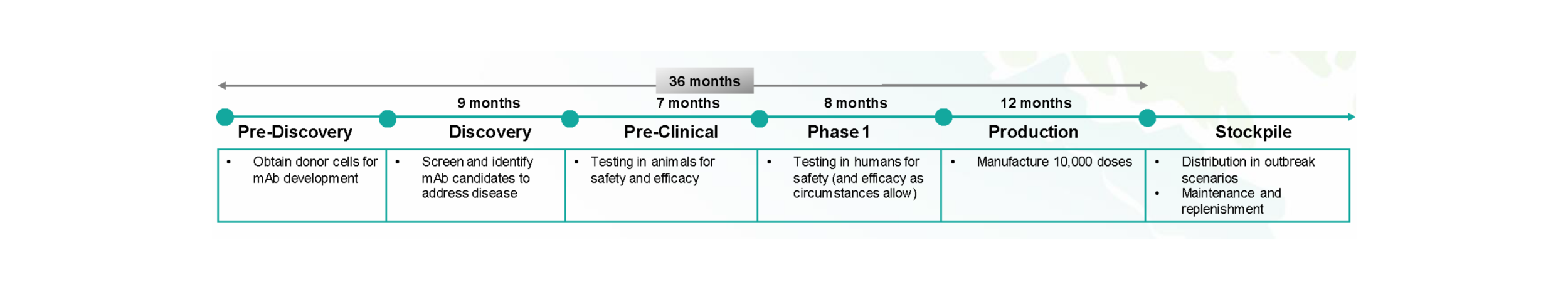
For the 100 target pathogens, the Center will advance mAbs through Pre-Discovery, Discovery, Pre-Clinical, and Phase 1 Clinical Trials and then manufacture approximately 10,000 doses to be stockpiled and readily available for emergency use should an outbreak occur. The figure below describes the individual phases within AHEAD100, including the upfront process to obtain materials and the scientific processes involved in identifying mAbs and developing them for animal testing. Subsequently, human trials follow, specifically for Phase 1, testing that the mAb solutions are safe (while gathering any efficacy data as available). Once safety is established, the mAbs will be produced for stockpiling and maintenance.
Figure 6: Subphases within AHEAD100 delivery with associated activities and durations, showing the 3-year end-to-end development cycle per mAb.
The Center will implement these plans as follows:
PRE-DISCOVERY activities will be conducted to identify and gather samples of donor cells across the globe. Center personnel will work with the appropriate regulatory personnel to confirm a regulatory process that supports the movement of these samples across borders to facilitate research and development of new mAbs.
DISCOVERY activities will be delivered by selected delivery partners through a network of leading academic, industry, and research participants. With input and guidance from the Scientific Advisory Committee, a target product profile will be determined for each pathogen. Pre-clinical activities will endeavor to discover the most potent and effective mAbs for each pathogen target, with a preference toward creating broad pan-family antibody and/or cocktail solutions where possible.
PRE-CLINICAL activities, including mouse and non-human primate studies, will be delivered by selected delivery partners through a network of leading academic, industry, and research participants. Critical dependencies will include BSL-4 lab capacity for testing mAbs for 16 pathogens.
PHASE 1 clinical testing will be performed to ensure safety and efficacy data is readily available to provide regulatory burden-of-proof in emergency approval situations. The Center will work with key experts and regulatory agencies, including the FDA, WHO, and others, to fully establish the regulatory pathways needed to define the primary, secondary, and exploratory endpoints required to address safety and efficacy concerns and enable a streamlined mAb EUA authorization as necessary.
PRODUCTION partners will be selected with proven CMC and GMP capabilities to ensure the highest degree of quality and regulatory compliance. A global network of manufacturing sites is intended to provide diverse supply, scalability, geopolitical risk management, and potential greenfield economic development opportunities. The Center will work with industry partners and CDMOs to define key manufacturing and production parameters for process development for multiple stages within the development, including controls for clinical Phase 1 trials and stockpile dose manufacturing (10,000 doses).
A STOCKPILE OF 10,000 DOSES of each developed mAb solution will be collected and maintained to enable rapid outbreak response. The Center will work with global public health agencies like the WHO and individual national governments to spread the stockpile of monoclonal antibodies regionally to enable enhanced access around the globe. The rationale behind stockpiling distribution is also driven by the need to mitigate risk from both natural and geopolitical disasters.
In addition to AHEAD100 delivery measures for known epidemic threats, the Center will also ESTABLISH A RAPID DEVELOPMENT & RESPONSE NETWORK capable of pivoting to a previously unidentified, new DISEASE X.
In close coordination and collaboration with the FDA and WHO, the Center would help shape and better define these regulatory pathways to enable rapid deployment of mAbs to prevent future outbreaks from becoming pandemics. The key processes to define and align with the FDA/WHO are the pre-emergency submission and approval processes and the associated outcomes necessary to provide for Emergency Use Authorization (at the time of an outbreak), thereby allowing the emergency deployment of mAbs targeting vulnerable and at-risk populations. The Center will initiate pre-emergency submissions with the U.S. FDA and WHO (including but not limited to pre-clinical and Phase 1 findings) at a minimum. Pre-emergency regulatory activities are expected to consider i) the assessment platform by the regulatory agency and a roster of experts, and ii) the mAbs product assessment and evaluation criteria. The outcome of a pre-emergency submission for emergency use should be to prepare an evaluation report and recommendation sufficient for the regulator to approve emergency use coincident with a Public Health Emergency (PHE) declaration when warranted. The WHO’s Emergency Use Listing Procedure (as amended in Version 13, December 2020) provides a template for pre-emergency regulatory reviews, and we would work with the WHO and others to consider the unique assessment and evaluation criteria applicable for mAbs. Such pre-emergency regulatory processes will be crucial to actualize on the ground the value of the stockpiled mAbs during the early stages of an outbreak to effect rapid containment, prophylaxis, and halt the outbreak from growing into an epidemic or pandemic.
A comprehensive roadmap has been built to address all 100 pathogens on the list over six years. A preliminary snapshot of the first two years, 2022 and 2023, of delivery within the AHEAD100 program is included here for a subset of pathogen families prioritized for initial delivery. (Figure 7: A description of a subset of delivery activities in 2022, associated pathogens, and durations spent in each phase; click to enlarge)
The 2022 launch projects and goals include:
Completing Discovery on seven additional mAb candidates.
Conducting Pre-clinical testing on three additional mAb candidates.
Advancing 6 “shovel ready” (discovery and pre-clinical phases complete) mAbs through Phase I trials.
Beginning Production for 10,000 dose stockpiles on eight mAbs.
Establishing a global network of manufacturing partners with capacity for mAb stockpile delivery by the end of 2022.
Coordinating engagement with regulators and agreeing to a framework for clinical trials sufficient to enable EUA approval in conjunction with development and manufacturing partners
Working with federal, state, and local authorities, healthcare systems, and nonprofits to identify the approach and requirements to protect the most vulnerable populations
Developing a detailed ecosystem integration and collaboration plan accounting for public health and defense requirements in one accord
Building the relationships and processes necessary for a capability demonstration, which will demonstrate the full-lifecycle end-to-end ability to mobilize rapid distribution and delivery of mAb for a target pathogen with a high probability of occurrence and collect data during deployment to demonstrate safety and efficacy.
By the end of 2023, the Center will begin stockpiling mAbs and complete its stockpile addressing the remaining pathogens by 2027. The AHEAD100 program will provide medical countermeasure coverage for a total of 90 viral and 10 bacterial pathogens. 73 of the 90 viral pathogens have been identified with detailed expert input from Dr. Crowe’s lab, with room to identify 17 new or emerging pathogens.
This delivery roadmap delivers the total stockpile at the end of 2027, prioritizing the most virulent diseases for delivery in the initial years. Stockpile completion milestones for pathogen families are detailed below.
Priority 2: Innovation
The Center’s ability to drive discovery, development, and distribution of mAbs with high efficacy, streamlined distribution, and low cost necessitates ongoing innovation in the science, technology, platforms, manufacturing, regulatory, talent, and delivery of mAbs solutions for infectious diseases.
The Center’s innovation objectives include:
Defining consistent mAbs development platforms for our R&D and investing in new platform technologies where needed.
Transitioning the mode of delivery of mAbs products from current prevalent intravenous methods to intra-muscular administration, simplifying administration, and reducing cost.
Collaborating with regulators to transform regulatory processes for product testing, trials, and approvals for emergency use of mAbs to treat and contain infectious disease outbreaks (to prevent these outbreaks from escalating to pandemics).
Advancing mAbs manufacturing technologies and platforms to reduce cost, improve quality, increase speed, and provide flexible capacity.
Developing and maintaining a rapid development platform consistent with developing needed mAbs solutions, consistent with the 100 Days Mission for a future “Disease X” to build capacity and capability to address emerging disease as necessary.
Facilitating cross-sector collaboration as a condition precedent in pandemic prevention processes and solutions
The Center will achieve these objectives by:
Investing in enabling efficiencies in known platforms to accelerate the development and manufacturing of mAbs. The Center will invest in available platform technologies to develop the majority of mAbs for its pathogen portfolio. This will ensure that mAbs can easily be scaled with existing capacity and platform technologies. In addition, leveraging these platforms will validate their use in an infectious disease context and promote their use for added indications.
Supporting the development of new platforms within mAbs discovery. The Center will support the development of technologies through its ecosystem to enable more efficient mAbs production. Examples of technologies like these include innovations that reduce time to manufacture, increase mAbs potency, and reduce the need for infrastructure like cold chain.
Developing customized regulatory pathways to support the use of an investigational stockpile. The use of a stockpile of investigational drugs is uncommon, and the Center will pioneer new regulatory pathways in conjunction with world regulatory agencies to enable successful deployment.
Delivering the portfolio of mAbs products intramuscularly or subcutaneously during outbreaks. The Center will support additional research and process scale-up associated with delivering mAbs intramuscularly or subcutaneously to ease the administrative costs and care associated with intravenous delivery.
Priority 3: Response
The ability to work in an integrated and coordinated manner with key global parties to aggregate capabilities and leverage them to deliver mAbs products for any known or unknown disease is instrumental to containing outbreaks. Close coordination will be required with entities that provide global monitoring and surveillance, diagnostics, regulatory pathways and approvals, response, vaccines, and therapies.
The Center’s response planning objectives include:
Building relationships and processes necessary for a capability demonstration, which will demonstrate the full-lifecycle end-to-end ability to mobilize rapid distribution and delivery of mAbs for a target pathogen with a high probability of occurrence, with the added objective of capturing clinical trial data within the context of an outbreak.
Establishing collaborations and partnerships with leading public and NGO health agencies to enable the Center’s mAbs products to be utilized as a rapid response solution to new global outbreaks, including creating a new regulatory pathway to deploy mAbs in the context of rapid response.
Working with public health, local authorities, and healthcare systems to establish processes and solutions that directly benefit the at-risk and underserved communities disproportionately impacted during a pandemic.
The Center will achieve these objectives as follows:
Planning and executing a capability demonstration focused on 1-2 mAb(s) for high recurrence (e.g., Rift Valley Fever, Nipah, Lassa) pathogens, whereby these processes can be tested, demonstrated, and documented with clinical trial infrastructure in “live” outbreak situations, and the broader value proposition for mAbs in pandemic prevention can be measured.
Building partnerships and working relationships with key entities in the pandemic response, including CEPI, FIND, Rockefeller Foundation, WHO, government agencies, and others, to enable a coordinated response as public health emergencies arise. Processes will be in place to respond to an outbreak, including the ability to expedite regulatory EUA approvals, mobilize global distribution of stockpiled mAbs, and to work with local health agencies to enable mAbs delivery. Beyond the initial outbreak response, process will be in place to perform Phase 2 clinical trials after mAbs administration, ramp-up additional manufacturing, and potentially other discovery and scientific research related to the outbreak.
Developing country-specific customized plans to facilitate the successful distribution of mAbs during outbreaks. The Center will drive the definition of country-specific plans with partner nations to enable pandemic preparedness, including the use of mAbs during emergencies, distribution, and scale-up.
Partnerships and working relationships will be established with key entities in the pandemic response ecosystem. Processes will be established to respond to an outbreak, including the ability to expedite regulatory EUA approvals, mobilize global distribution of stockpiled mAbs, work with local health agencies to enable mAbs delivery, initiate additional clinical trials and testing after mAb administration, ramp-up additional manufacturing, and potentially other discovery and scientific research related to the outbreak. In addition, a capability demonstration will be performed, focused on 1-2 mAb(s) for high recurrence pathogens, whereby these processes can be tested and demonstrated in “live” outbreak situations, and the broader value proposition for mAbs in pandemic prevention can be measured.
Funding
The Center is structured as a nonprofit public-private partnership and will receive funding from philanthropic organizations, governments, and other NGOs. With a strong need for U.S. government (USG) leadership and investment, the Center seeks approximately 75% of the AHEAD100 program investment from USG sources. In addition, other governments are expected to provide ongoing funding beyond the initial AHEAD100 project. The next phase of work will determine the most appropriate means by which foreign governments will participate.
The Center will pursue a variety of funding channels for AHEAD100, which may include programmatic USG funding via relevant agencies; philanthropic/NGO funding of AHEAD100 overall, specific projects, pathogens, phases, or outcomes; industry investment or in-kind support contributions; and other sources.
To achieve its strategic priorities, the Center will: establish a network of collaboration partners and advisors, create an external affairs function focused on building and maintain strategic and operational partnerships, establish a regulatory management function, and engage relevant government agencies to develop both advisory and ongoing working relationships.
To elevate national and global adoption of mAbs usage in the context of pandemic rapid response, the Center will pursue a media strategy focused on amplifying key voices, highlighting eminent academics, scientists, policymakers, and industry representatives. This will be achieved through a national and regional marketing strategy to amplify the Center’s mission and progress with key stakeholders.
Marketing
Governance

The Center’s Board of Directors governs the Center. A CEO-led management team coordinates the day-to-day operations with an organizational headquarters in Montgomery County, Maryland, supported by distributed capacities worldwide. In addition, an Advisory Committee advises the Center on scientific and other pandemic prevention matters across the lifecycle of mAbs development and pandemic preparedness and response. Initially, the Strategy Phase Steering Committee will serve as the Advisory Committee until the Center’s permanent governance is fully established.
The Board of Directors represents the Center’s highest body and is informed by the Advisory Committee. The Board will comprise 11 voting members and is the Center’s highest decision-making authority. Sub-committees within the board provide the Executive Management team input on specific issues. The entire Board is planned to be operational in the 4th quarter of 2021.
A Donor Council will be established in the 4th quarter of 2021 and will function in an advisory capacity and receive updates about organization operations from the Board.
The Advisory Council comprises key cross-sector and scientific leaders representing global pandemic prevention coordination and leading related innovations. The Advisory Council will also hold responsibility for helping to advance the interests and needs of the most vulnerable populations in pandemic prevention.
The Project Review and Coordination Committee provides governance around Center initiatives & their related projects.
Figure 8: The Center's governance model ensures that the best of technology and science is brought to the pandemic prevention ecosystem while ensuring that benefits are distributed equitably around the globe.
Board of Directors
The Board is the Center's governing body and has final authority for all funding, policy, and product development decision-making for the Center operations.
Key functions of the Board include:
Enabling Executive Management to efficiently execute the objectives of the Center and the instructions and decisions of the Board.
Confirming that the CEO and team are managing the organization in line with the legal and regulatory requirements & the Board Charter.
Setting policies and principles for pursuing the organization’s mission.
Approving budgets, investments, and business plan updates
Providing governance and fiduciary oversight to the organization's activities, finances, and performance.
Assisting management with the development and maintenance of key public and private sector relationships
The Board will comprise 11 voting members, with the option to include non-voting members as appropriate. All 11 members will be appointed to serve 3-year terms, which can be renewed, as approved by the Board and described in the Center’s bylaws. The Board will constitute specific sub-committees at the direction of the Chair as necessary to address particular topics.
Donors’ Council
The Donors’ Council is an advisory body that provides input and guidance to the Board on strategic matters. Decision-making powers reside with the Board, per the Center’s bylaws.
Key functions of the Donors’ Council will be to:
Select a Chair to represent donors for a term of 2 years
Receive regular updates from Executive Management & provide managerial and operational guidance
Support the Center with active outreach to potential donors and partners
All participants who have provided a financial contribution greater than $150,000 over the prior 18 months or above a total threshold amount as determined by the Board will be invited to join the Donors’ Council. The Council will treat each representative without regard to the size of the representative’s donation. The Council will include a mix of philanthropic foundations, government agencies, and corporate and individual donors.
Advisory Council
The Advisory Council is the principal advisory body to the Board on strategic, policy, and technical matters. It works with Executive Management to remove any programmatic roadblocks and drive equitable access.
Key functions will be to:
Advise on prioritization of new pathogens to be considered for inclusion within the AHEAD100 program.
Advise on target product profiles, platform technologies, innovations, etc.
Review expressions of interest and assist in the partner shortlisting.
Review scientific and development progress of initiatives and reviews the quality of the scientific advancement of initiatives.
Work with critical external global stakeholders to address challenges in the key domains such as R&D, manufacturing & quality control, stockpiling, regulatory, IP rights, & product delivery.
Request and leverage support of the Executive Management Team and Scientific Advisory Committee to address outlined challenges.
Advise and support the Center’s mission to enable rapid response globally in the event of outbreaks through international diplomacy and governmental/non-governmental stakeholder connections.
Advise on the priorities and strategies for address vulnerable populations throughout the solution lifecycle
Advise and support fair and equitable access of products for low- and middle-income countries in the event of an outbreak or pandemic.
Operations
Financials
The six-year program cost estimate to deliver AHEAD100 is $2.5B. This includes developing approximately 65 total mAbs solutions, with 22 mAbs supporting prophylactic or therapeutic action across multiple pathogens.
The key cost drivers are:
Click to view a graphical breakdown of key cost categories associated with the operationalization of the Center and its flagship initiative, AHEAD100, reveals the significant contributors to be discovery, development, and stockpiling cost.
Programmatic costs ($2.4B, ~96% total cost) associated with implementing and executing AHEAD100, including Development and Stockpile, Innovation, Capability Demonstration, and Global Process Development initiatives.
Enabling costs ($21M, ~1% total cost) associated with management and administration of the Center.
Back-office costs ($75M, ~3% total cost) associated with the Center's day-to-day operations, including HR, Finance, IT, data and analytics, legal, and audit/compliance.
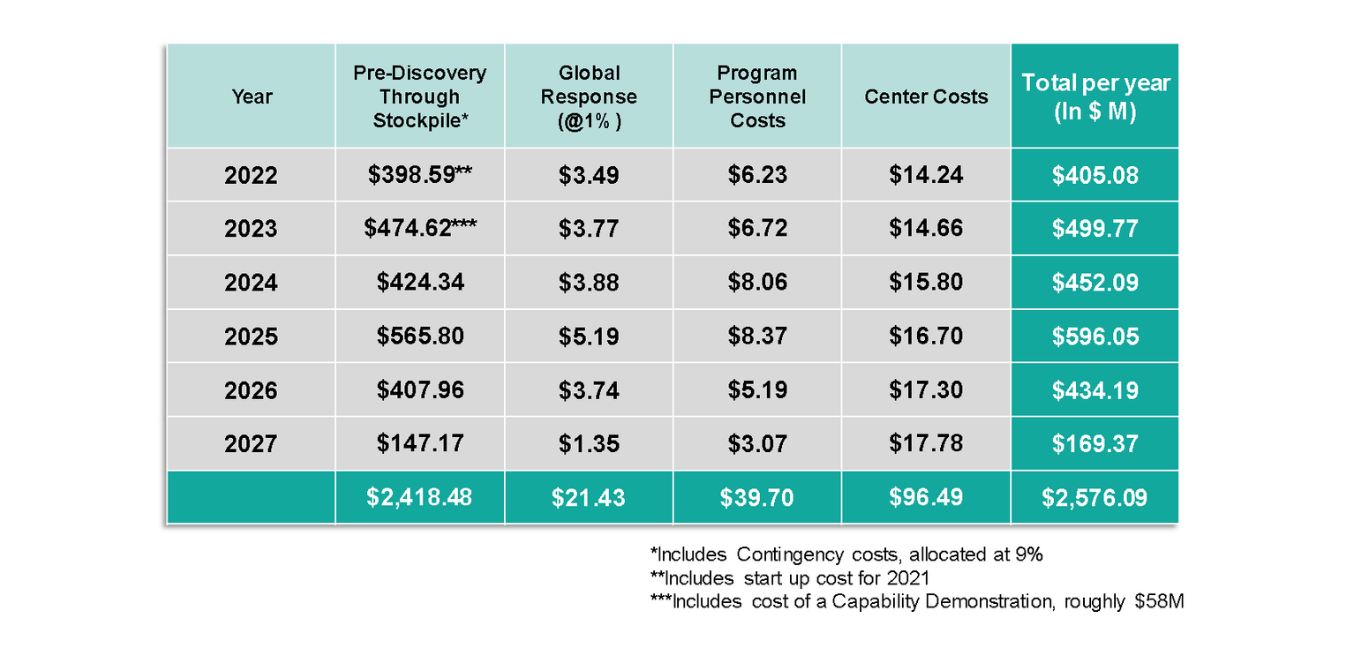
A cash flow analysis of the program cost requirement reveals the following annual outlays:
Table 2: Annual cash outlays to support Center operations and program execution for AHEAD100. Note that Contingency cost is included within Center costs).
Next Phase
The Center will officially launch as a 501(c)3 independent legal entity and charitable organization in the third quarter of 2021. The organization will initially be developed around key programmatic and technical capabilities needed to support mAbs delivery, the integration of mAbs into global pandemic response processes, and enabling functions like funding and donor management.
Key activities planned for the remainder of 2021 include i) identifying and seating the Board of Directors, CEO, and other key management positions, ii) transitioning the Strategy Phase steering committee to become the initial Advisory Council for the Center, iii) establishing a headquarters location in Montgomery County, MD, iv) selecting delivery partners for initial mAbs delivery efforts, v) securing funding to launch delivery efforts on first mAbs delivery projects, vi) positioning for government, NGO, and philanthropic funding for the long term delivery of AHEAD100 and sustainable operations of the Center.
The Center will also implement a rigorous program management approach to supervise milestones and delivery against target timelines defined for the AHEAD100 roadmap.
Click to view figure 10: Summary of launch activities for the Center.
An endeavor of this scale is not possible through independent effort. Accordingly, the Center will define and operationalize an External Affairs function that gathers strategic and operational partnerships to aggregate funds and collate together an ecosystem that spans from surveillance to last-mile distribution to enable the effective use of mAbs in an outbreak. This function will include personnel focused on engaging directly with the governments (explicitly focusing on the U.S.), the NGO landscape, and donors. A summary of launch activities for the Center for 2021 and 2022 is depicted to the right.
Key Risks

RISKS & MITIGATIONS
Waning public interest
Align strong voices from key ecosystem providers
Maintain assertive marketing campaign
Help develop public champions for pandemic prevention
Funding limitations
Structure $2.5B to be delivered in phases as major milestones are accomplished
Develop long-term approach based on diversified global funding
Ecosystem collaboration
Maintain transparency and visible support for collective cross-sector benefits
Capacity & supply chain constraints
Create formal collaborations where key scientific constraints exist
Utilize Center to develop critical path/ingredient limitations with ecosystem plan to resolve
Regulatory constraints
Transform pathways necessary to obtain EUAs in collaboration with key agencies
Marketing and PR campaign to enhance understanding and benefits on mAbs
As with any endeavor, associated risks can delay or destabilize the Center or its implementation of AHEAD100. Predicting and accounting for possible risks will help with program planning, execution, transparency, and accountability. The sections below summarize the projected risks of the program and initial mitigation strategies. Once operational, the Center will incorporate standard identification, measurement, and mitigation practices as an ongoing common risk management practice within its program management office. Risk management will be a function of the program management office and will be regularly addressed by management and the Board of Directors.
Waning Public Interest
Public interest and government funding priorities for pandemic prevention and response fade as outbreaks pass, and SARS-CoV-2 is likely no exception. The Center will coordinate with government leaders across the value chain from research to delivery and distribution to drive continued advancement of solution development and deployment focused on future pandemics. The External Affairs function of the Center is explicitly chartered for this purpose to drive sustained communications, education, awareness, and engagement and interactions with key government agencies, including the NIH, BARDA, and FDA.
Funding Limitations
As evidenced throughout this document, the endeavor to stockpile mAbs at this scale is a capital-intensive process that requires an estimated $30 million per mAb. To build a portfolio, the Center will require consistent, sustained funding over six years. The Center has already developed a variety of relationships with key national and global philanthropic organizations along with the NGOs and government agencies and will continue to:
Seek opportunities to build a high-quality funding portfolio
Offer a variety of ways to engage and fund contributions, with the option to contribute to specific elements within the value chain (i.e., Phase 1 clinical trials or manufacturing) or support end-to-end deployment by a particular pathogen in alignment with organizational missions
Provide options to engage as a strategic partner, with longer durations, allowing network partners to co-create strategies and implementation plans, spurring more significant investment
In addition, the success of the Center’s initiatives depends on political relationships within the U.S. and in other countries, as funding priorities change over time. The Center will build a portfolio of relationships with governments through direct engagement, multilateral arrangements, and other routes for engagement to strengthen the strategic and operational partnerships needed to execute as outbreaks occur.
Ecosystem Collaboration
As SARS-CoV-2 has demonstrated, the challenges of mounting a coordinated response to a disease outbreak are significant. Priorities across nations, amongst governments, within government agencies, across public-NGO-private sector boundaries, and even among the scientific communities can often differ or be at odds. Despite best intentions, any single entity cannot mount a coordinated, collaborative response to future outbreaks. It will require the collective power and will of government-NGO partnerships, supported by philanthropic endeavors, to plan and orchestrate effective response processes to future outbreaks. The Center will partner with others to create a broad-based “coalition of the willing” that ensures effective response processes are in place and ready to implement for the next outbreak.
Supply Chain & Capacity Constraints
The operational capacity and support required for a program of this scale are significant. To enable delivery on time and within budget to meet strategic plan objectives, the Center will leverage capacity and disperse specific programs for development across BSL-4 laboratories (which allow work on highly virulent pathogens), contract development manufacturing organizations (CDMOs), contract research organizations (CROs), and industry partners. The Center will establish an active ecosystem of partners to distribute and allocate RFPs for program development to expand and retain capacity needs.
Regulatory Constraints
To create and dispense the stockpile, the Center will require regulatory pathways that allow for emergency use in the event of an outbreak. The Center will actively engage and collaborate with regulators, including the FDA, WHO, and other regional and local health agencies, to explore pathways that consider needs during epidemic outbreaks.
Limited Understanding of mAbs
Currently, vaccines are known as the primary solution for pandemic prevention and response. The Center will partner actively with leading entities like CDC, FDA, NIH, CEPI, WHO, and international governments to drive forward the value proposition of mAbs as a bridge therapy between the time of diagnosis and vaccine readiness.
“Our dream is that we don’t have pandemics in the future… but we need to build capacity to respond quickly to local outbreaks and contain them so they do not spread.”
— Dr. James Crowe, Founder, AHEAD 100